Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the Contact Us page.
Laminated Root Rot
Phellinus weirii (Murrill) R. L. Gilbertson
(= Poria weirii (Murill) Murill)
(=Inonotus weirii (Murrill) Kotl. & Pouzar
Basidiomycotina, Aphyllophorales, Polyporaceae
Hosts: Two forms of disease caused by Phellinus weirii are recognized, the Douglas-fir and cedar forms, based on cultural characteristics, symptomology, and host preference. It is now generally thought that these "types" represent two different taxa and should have separate names, but more research is required before the nomenclature is finalized. Hosts reported for the Douglas-fir form of Phellinus weirii are listed below.
|
Highly Susceptible |
Susceptible |
Tolerant |
Resistant |
|---|---|---|---|
|
Douglas-fir * |
California red fir |
Lodgepole pine * |
Yellow cedar |
|
Grand fir * |
Engelmann spruce * |
Ponderosa pine * |
Incense- |
|
Mountain hemlock |
Giant sequoia |
Sugar pine |
Redwood |
|
Pacific silver fir |
Noble fir |
Western white pine * |
Western redcedar |
|
White fir |
Pacific yew |
||
|
Sitka spruce * |
|||
|
Subalpine fir * |
|||
|
Western hemlock * |
|||
|
Western larch* |
*Hosts reported for B.C. Others are from elsewhere in North America.
The cedar form occurs as a butt rot in western redcedar, and on Alaska yellow cedar at high elevations at the coast. Deciduous tree species are immune to both forms.
Distribution: This form is widely distributed throughout the range of Douglas-fir in the province.
Identification: Infected trees may be randomly dispersed throughout a stand or may occur grouped in "disease centers," which are often visible from the air as openings (Fig. 6a). These often appear as patches of young regenerating conifers or resistant hardwood vegetation. The first crown symptoms, a retardation of height and branch growth followed by thinning and yellowing of the foliage are usually not evident until the root system is in an advanced stage of decay. Frequently a stress-induced cone crop will be formed at this stage. The bark of the lower bole sometimes has a darkened, water-stained appearance shortly before or after tree death (Fig. 6b). Basal resinosis is rare. Diseased trees with advanced root decay are frequently windthrown, producing typical "root balls" (Fig. 6c). White to tawny to mauve mycelium (ectotrophic mycelium) can usually be found on or in bark at the root collar and on the roots, particularly in mineral soil. A brown, crust-like mycelial growth often occurs growing over the ectotrophic mycelium on or near the root collar, on infected roots, and on exposed advanced decay (Fig. 6d). This crust like mycelium has the appearance and texture of blistering paint. The rarely produced annual fruiting bodies are brown, crust-like layers on upturned roots and on the underside of decayed logs (Fig. 6e). When fresh, they are light buff with narrow white margins; they turn a uniform dark brown when old and may remain in place for 2-3 years. The exposed surface of the fruiting body is poroid; the pores are small and somewhat irregular in outline.
The early stage of decay sometimes appears as a red-brown stain visible on fresh stump tops or cross sections of major roots (Fig. 6f); infection rarely extends more than 1 m up the stem in living trees. Later, stained wood becomes soft and the annual rings separate to form sheets or a typical laminated decay (Fig. 6g); numerous small pits can be seen in the decayed wood. Accumulations of mycelium with reddish-brown setal hyphae (Fig. 6h) usually form between sheets of decayed wood.
Damage: Laminated root rot poses a major threat to its most economically important host, second-growth Douglas-fir. The disease causes root decay, which can cause significant growth reduction, and makes trees susceptible to blowdown and stem breakage (the latter is rare in coastal trees).
Remarks: Initial infection and spread of the fungus in a stand occurs when healthy roots come in contact with diseased roots. The fungus can remain viable in stumps and roots for many decades after tree death providing thus serving as a source of inoculum for subsequent rotations. Mycelia do not grow freely through the soil and spores are not believed to be important in disseminating the disease. "Disease centers" develop around infected stumps and expand radially at rates of about 30 cm per year.
Distribution: Phellinus weirii causes a root and butt rot of western redcedar, particularly in old-growth trees throughout the Interior Cedar Hemlock biogeoclimatic zone. It is found to a limited extent on yellow cedar at higher elevations in coastal regions.
Identification: The early stage of decay appears as a yellow-brown stain. Later a laminate, pitted decay is formed (Fig. 6i). Fruiting bodies on cedar are perennial and are typically darker in colour. The pore surface tends to be somewhat smoother and pores are more regular than those found in fruiting bodies of the Douglas-fir form.
Damage: Western redcedar is rarely killed by P. weirii, damage being confined mostly to butt rot, which can extend 2-3 m up the bole of living trees; in cases of severe decay, up to 10 m. Most old-growth cedar has some degree of butt rot, much of which is caused by P. weirii. This can weaken the tree and lead to stem breakage low on the bole (Fig. 6j).
Microscopic Characteristics: Contextual hyphae thin-walled with frequent branching, simple septate, setal hyphae. Basidiospores hyaline, ovoid, smooth, negative in Melzer's, 4.5-6 x 3.5-4.5 µm. Growth in culture moderately rapid, mat white, becoming brown, laccase positive, simple septate, setal hyphae (Fig. 6j) averaging 350 µm (Douglas-fir form), 290 µm (cedar form). Stalpers: 1 3 4 (7) (8) 12 (14) 21 22 30 (31) (33) (34) 35 (37) 48 52 53 54 67 69 83 90. Some culture characteristics differ between the two forms of P. weirii. Cedar-type cultures grow slower, and are darker in colour; setal hyphae are shorter and narrower.
References:
Larsen, M. J., F. F. Lombard, and J. W. Clark. 1994. Phellinus sulphurascens and the closely related P. weirii in North America. Mycologia 86:121-130.
Morrison, D. J., M. Merler, and D. Norris. 1992. Detection, recognition, and management of Armillaria and Phellinus root diseases in the southern interior of British Columbia. Can. For. Serv., B.C. Min. For. FRDA Rep. No. 179.
Thies, W. G. and R. N. Sturrock. 1995. Laminated root rot in western North America. USDA For. Serv., CFS Res. Bull. PNW GTR 349.
Wallis, G. W. 1976. Phellinus (Poria) weirii root rot detection and management proposals in Douglas-fir stands. Can. For. Serv., For. Tech. Rep. No. 12.
Figures
Click on any image to see the full size version.
Press "Back" on your browser to return to this screen.

Figure 6a: Dead and dying trees in a P. weirii disease center.
 Figure 6b: Bark stain on Douglas-fir.
Figure 6b: Bark stain on Douglas-fir.
 Figure 6c: Windthrown Douglas-fir in a root-rot center with characteristic root-balls.
Figure 6c: Windthrown Douglas-fir in a root-rot center with characteristic root-balls.
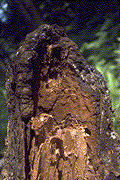 Figure 6d: Mauve-coloured ectotrophic mycelium and brown crust-like mycelium on a Douglas-fir root.
Figure 6d: Mauve-coloured ectotrophic mycelium and brown crust-like mycelium on a Douglas-fir root.
 Figure 6e: Fruiting body of P. weirii on Douglas-fir.
Figure 6e: Fruiting body of P. weirii on Douglas-fir.
 Figure 6f: Incipient decay in outer sapwood.
Figure 6f: Incipient decay in outer sapwood.
 Figure 6g: Laminated decay typical of P. weirii.
Figure 6g: Laminated decay typical of P. weirii.
 Figure 6i: Laminated decay in western redcedar.
Figure 6i: Laminated decay in western redcedar.
 Figure 6j: Stem breakage on western redcedar caused by P. weirii butt rot.
Figure 6j: Stem breakage on western redcedar caused by P. weirii butt rot.
 This Web page has been archived on the Web.
This Web page has been archived on the Web.